| Catalog No. | HT073036 |
|---|
| Species reactivity | Human |
|---|
| Applications | ELISA, Bioactivity: FACS, Functional assay, Research in vivo |
|---|
| Host species | Human |
|---|
| Isotype | IgG1-nd |
|---|
| Expression system |
Mammalian Cells |
| Clonality |
Monoclonal |
| Target |
ANG-2, ANGPT2, Angiopoietin-2 |
| Endotoxin level |
Please contact the lab for this information. |
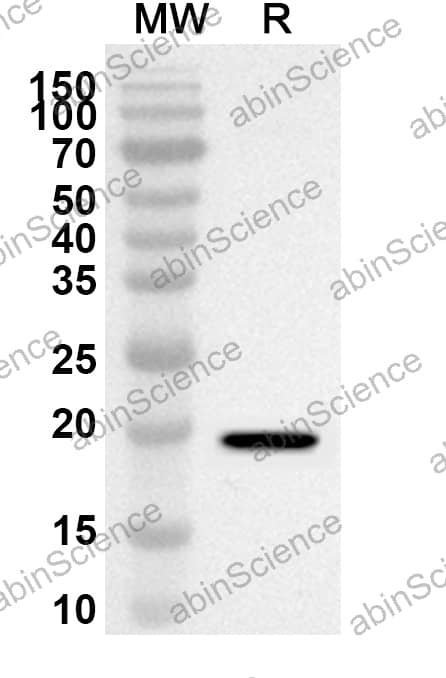
| Purity |
>95% purity as determined by SDS-PAGE. |
| Purification |
Protein A/G purified from cell culture supernatant. |
| Accession |
O15123 |
| Form |
Liquid |
| Storage buffer |
0.01M PBS, pH 7.4. Please refer to the specific buffer information in the hardcopy of datasheet or the lot-specific COA.
|
| Stability and Storage |
Use a manual defrost freezer and avoid repeated freeze-thaw cycles. Store at 4°C for short-term storage (1-2 weeks). Store at -20°C for up to 12 months. For long-term storage, store at -80°C. |
| Alternate Names | REGN-910, 1296818-77-3 |
|---|
| Background | Trebananib (2xCon4C; AMG 386) is an Fc fusion peptibody that prevents Tie2 receptor activation through binding of both angiopoietin 1 (Ang1) and Ang2. Trebananib has anti-angiogenesis activity.
• Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology., PMID:31108880
• Acute respiratory distress syndrome., PMID:30872586
• Faricimab Treat-and-Extend for Diabetic Macular Edema: Two-Year Results from the Randomized Phase 3 YOSEMITE and RHINE Trials., PMID:38158159
• Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): two randomised, double-masked, phase 3, non-inferiority trials., PMID:35085502
• Efficacy and Safety of Faricimab for Macular Edema due to Retinal Vein Occlusion: 24-Week Results from the BALATON and COMINO Trials., PMID:38280653
• TENAYA and LUCERNE: Two-Year Results from the Phase 3 Neovascular Age-Related Macular Degeneration Trials of Faricimab with Treat-and-Extend Dosing in Year 2., PMID:38382813
• Simultaneous Inhibition of Angiopoietin-2 and Vascular Endothelial Growth Factor-A with Faricimab in Diabetic Macular Edema: BOULEVARD Phase 2 Randomized Trial., PMID:30905643
• Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials., PMID:35085503
• Blocking the angiopoietin-2-dependent integrin β-1 signaling axis abrogates small cell lung cancer invasion and metastasis., PMID:38775153
• Loss of TGFβ-Mediated Repression of Angiopoietin-2 in Pericytes Underlies Germinal Matrix Hemorrhage Pathogenesis., PMID:39129597
|
|---|
| Note |
For research use only. Not suitable for clinical or therapeutic use. |

 中文
中文 English
English