In October, several breakthrough studies powered by abinScience and focused on antibody and protein research were published in top journals such as Pharmaceuticals and Cell Reports, covering critical areas including intervertebral disc degeneration, cancer signaling, and Alzheimer's disease. The high-purity recombinant proteins and antibodies provided by abinScience have been instrumental in supporting core research in disease mechanisms and target screening, enabling key breakthroughs. In this literature update, let's review these significant advances from frontline laboratories.
Title: Methoxypoly(ethylene glycol)-poly(salicylic acid) nanoparticles alleviate intervertebral disc degeneration by inhibiting ferroptosis of nucleus pulposus cells
Journal: Chemical Engineering Journal
Impact Factor: 13.2
Affiliation: Department of Orthopedics, Peking University Third Hospital
Intervertebral disc degeneration (IDD) is the primary cause of low back pain (LBP), the leading global cause of disability. Current treatments only relieve symptoms rather than reverse pathology. Ferroptosis, an iron-dependent form of cell death, drives early IDD through lipid peroxidation and inflammatory factors, leading to loss of nucleus pulposus cells (NPCs) and extracellular matrix (ECM) degradation. Salicylic acid (SA) has anti-inflammatory activity but poor bioavailability. Methoxypoly(ethylene glycol)-poly(salicylic acid) nanoparticles (mPEG-PSA NPs) prepared via ring-opening polymerization-induced self-assembly (ROPISA) enable localized high-concentration delivery. This study used mPEG-PSA NPs to alleviate IDD by inhibiting ferroptosis in NPCs. Results showed that mPEG-PSA NPs significantly improved disc height index, reduced ECM degradation, and suppressed ferroptosis in NPCs. Mechanistically, they activated the Hippo pathway, with RNA-seq revealing downregulation of ferroptosis-related genes. The study concluded that mPEG-PSA NPs offer a novel nanotherapeutic strategy for IDD via Hippo/YAP-mediated anti-ferroptotic effects, with strong clinical translation potential.
abinScience provided the Anti-Human GAPDH Monoclonal Antibody (1A200) (Cat. No.: HY030035) for this study, used as an internal control in Western blot experiments to quantify protein expression levels of Aggrecan, MMP13, LATS1, phosphorylated LATS1, YAP, and phosphorylated YAP, thereby validating the activation of the Hippo/YAP pathway and the protective effects of mPEG-PSA NPs in IDD.
Title: ROR2-specific CAR-T cells are effective against hematologic and solid tumors and well tolerated in mice
Journal: Cell Reports
Impact Factor: 10.6
Affiliation: University Hospital Würzburg
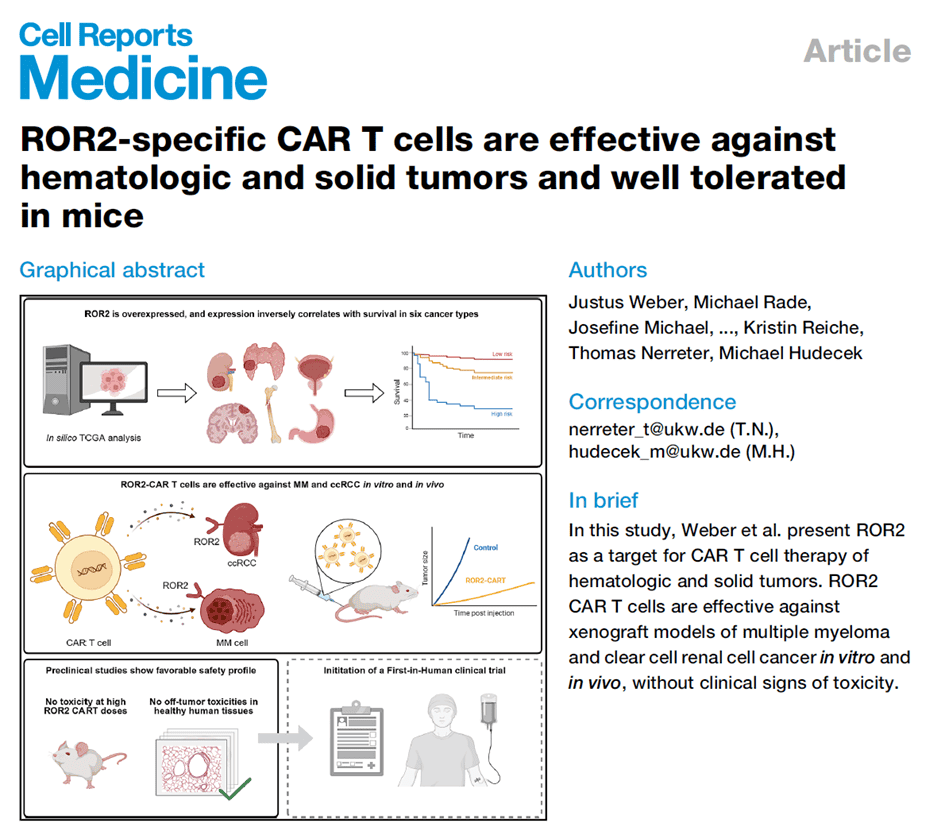
This study explored receptor tyrosine kinase-like orphan receptor 2 (ROR2) as a target for chimeric antigen receptor (CAR) T-cell therapy in hematologic and solid tumors. ROR2 plays a role in cancer signaling and is associated with tumor invasiveness and metastasis. It is highly expressed in cancers such as multiple myeloma (MM) and clear cell renal cell carcinoma (ccRCC) but restricted in healthy adult tissues, resembling a cancer-fetal antigen. Using flow cytometry and dSTORM microscopy, ROR2 expression was assessed. Second-generation ROR2-CAR T cells were constructed and tested for in vitro cytotoxicity, cytokine secretion, and proliferation, as well as in NSG mouse xenograft models for efficacy and toxicity. Results showed ROR2 expression in 82% of primary MM patient cells. ROR2-CAR T cells demonstrated specific killing activity against MM and ccRCC cell lines and primary cells, significantly inhibiting tumor growth and extending survival in vivo. Public database analysis revealed high ROR2 expression correlated with poor survival in six cancers, particularly ccRCC. High-dose ROR2-CAR T infusion showed no on-target off-tumor toxicity, with no clinical or histopathological evidence of harm. The study concluded that ROR2 is an ideal CAR-T target for MM and ccRCC, supporting its potential as a cancer-fetal antigen, with plans to initiate clinical trials.
abinScience provided the Mouse IgG1 Isotype Control antibody (Cat. No.: MV080107) and scFv isotype control antibody (HyHEL-10) (Cat. No.: YP293013). These control antibodies were used in tissue cross-reactivity (TCR) experiments to ensure IHC staining specificity, a critical component in confirming low ROR2 expression in normal tissues and absence of off-target toxicity, supporting the safety evaluation of ROR2-CAR T cells.
Title: Galectin-9 activates the TLR4-NLRP3 inflammasome pathway and promotes tau pathology in Alzheimer's disease
Journal: Brain, Behavior, and Immunity
Impact Factor: 7.6
Affiliation: Department of Neurology, Renmin Hospital of Wuhan University
This study investigated the role of Galectin-9 (Gal-9) in Alzheimer's disease (AD) pathology, characterized by tau deposition, microglial activation, and neuroinflammation. Gal-9, a β-galactoside-binding protein secreted by activated microglia, is elevated in AD patient brain tissue and cerebrospinal fluid and correlates with cognitive impairment. The study found that tau fibrils promote Gal-9 expression and secretion in microglia, observed similarly in tau P301S transgenic mice. Gal-9 binds toll-like receptor 4 (TLR4) on microglia, activating the NLRP3 inflammasome and releasing pro-inflammatory cytokines IL-1β and IL-18, which are toxic to neurons and induce tau hyperphosphorylation and synaptic damage. Knocking out Gal-9 reduced microglial activation, tau pathology, synaptic degeneration, and cognitive deficits in tau P301S mice, and inhibited tau pathology spread induced by tau fibril injection. Conversely, Gal-9 injection exacerbated these pathologies. The study concluded that the Gal-9-TLR4-NLRP3 axis mediates microglia-neuron interactions and is a key mechanism in tauopathy, suggesting it as a potential therapeutic target for AD.
abinScience provided the Gal-9 protein (Cat. No.: HT173011) for treating BV2 cells. It was used to study the time-dependent effects of Gal-9-TLR4 binding on downstream pathway protein levels and to verify whether Gal-9-induced inflammation is mediated via TLR4. As a key experimental reagent, Gal-9 supported validation of its role in activating the TLR4-NLRP3 inflammasome and promoting tau pathology, forming a critical part of elucidating Gal-9 as a therapeutic target in AD.
Title: Ligusticum chuanxiong Hort. Targets hsa-miR-10a-5p to Potentially Induce Apoptosis and Modulate Lipid Metabolism in Glioblastoma: A Natural-Product-Based Therapeutic Strategy
Journal: pharmaceuticals
Impact Factor: 4.8
Affiliation: The Chinese University of Hong Kong

Glioblastoma (GBM) is the most common aggressive primary malignant brain tumor with a dismal prognosis—2-year survival is only 18–33%. Treatment faces challenges from drug resistance and recurrence. Metabolic reprogramming (e.g., lipid metabolism) and extracellular matrix remodeling (e.g., collagen deposition) play key roles in tumor progression. The traditional Chinese herb Ligusticum chuanxiong is used to promote blood circulation and has shown anti-glioma activity, but its molecular mechanisms—especially interactions with miRNA networks—remain unclear. This study explored how Ligusticum chuanxiong Hort. targets hsa-miR-10a-5p to potentially induce apoptosis and modulate lipid metabolism in GBM, proposing a natural-product-based therapeutic strategy. Results showed that Ligusticum chuanxiong granule (CXG) solution dose-dependently inhibited cell proliferation, promoted apoptosis, and suppressed extracellular matrix organization. Mechanistically, CXG downregulated hsa-miR-10a-5p, which targets BCL2L11 to induce pro-apoptotic effects and forms a coherent feed-forward loop with transcription factors SREBF1 and E2F1 to inhibit lipid metabolism. EMSA confirmed E2F1 binding to the hsa-miR-29a promoter, leading to cooperative suppression of hsa-miR-29a-3p by SREBF1 and E2F1. Ferulic acid and adenosine in Ligusticum chuanxiong may regulate the EGFR-E2F1-hsa-miR-10a-5p axis. The study highlighted the multi-target anti-GBM mechanism of Ligusticum chuanxiong and proposed a new strategy combining metabolic intervention with miRNA-targeted therapy, offering fresh insights into feed-forward loop regulation in miRNA networks.
abinScience provided the E2F1 protein (Cat. No.: HX082022) for EMSA binding reactions to verify whether E2F1 binds the hsa-miR-29a promoter region, confirming its transcriptional repressive role in the miRNA regulatory network. Validating direct E2F1-miR-29a promoter binding was a key part of elucidating how CXG solution inhibits lipid metabolism via the SREBF1/E2F1-miRNA axis, supporting experimental evidence for its overall multi-target anti-GBM activity.
Literature Citation Reward Program
To thank researchers for their support and trust in abinScience, we are excited to launch the Literature Citation Reward Program! Whether you're a pioneer in life sciences or a dedicated lab explorer, if you publish in an SCI journal using any abinScience product, you're eligible for generous rewards!

 中文
中文 English
English






