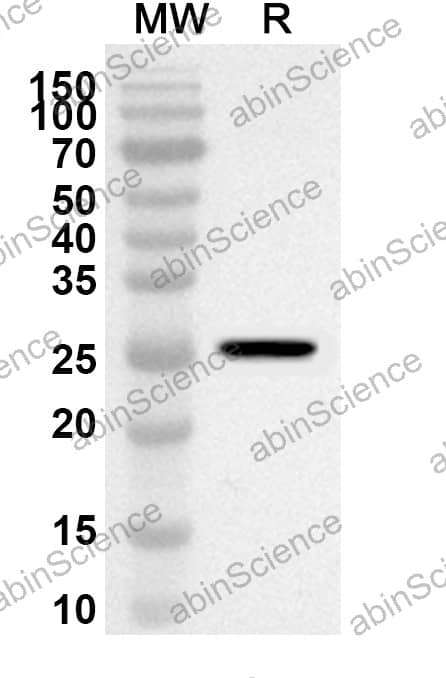
| Background | Certolizumab is a Fab fragment of a monoclonal antibody to human tumor necrosis factor alpha (TNFα) which has potent antiinflammatory activity and is used in the therapy of severe rheumatoid arthritis and inflammatory bowel disease. Antibodysystem Anti-Certolizumab Antibody, pAb, Rabbit is produced from sera of a rabbit immunized with Certolizumab.
• Certolizumab pegol, abatacept, tocilizumab or active conventional treatment in early rheumatoid arthritis: 48-week clinical and radiographic results of the investigator-initiated randomised controlled NORD-STAR trial., PMID:37423647
• Certolizumab Pegol: A Review in Moderate to Severe Plaque Psoriasis., PMID:32207094
• Certolizumab Pegol in Plaque Psoriasis: Considerations for Pregnancy., PMID:33769772
• Certolizumab treatment of localized pyoderma gangrenosum in a pregnant patient., PMID:37905433
• Certolizumab pegol for hidradenitis suppurativa: Case report and literature review., PMID:33135826
• Certolizumab pegol for induction of remission in Crohn's disease., PMID:31476018
• ACG Clinical Guideline: Management of Crohn's Disease in Adults., PMID:29610508
• [Certolizumab]., PMID:31208736
• Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation., PMID:27140438
• Certolizumab pegol for the treatment of psoriatic arthritis and plaque psoriasis., PMID:31917928
|
|---|

 中文
中文 English
English