In April, multiple studies focusing on antibody and protein research were published in top-tier journals such as Nature and Cell, covering areas including targeted cancer therapies, antiviral drug development, and exploration of radiolabeled antibodies. We are delighted to note that some of these achievements were supported by abinScience's protein and antibody products. In this literature roundup, we highlight several representative research advancements. Join us in exploring these recent scientific breakthroughs worth noting.
Title: Rabies virus large protein-derived T-cell immunogen facilitates rapid viral clearance and enhances protection against lethal challenge in mice
Journal: Nature
Impact Factor: 5.4
Author Affiliation: Zhongshan Hospital, Fudan University
This study developed a novel dual-immunogen mRNA rabies vaccine, RABV-G-LT, combining the viral glycoprotein RABV-G and a T-cell immunogen, RABV-LT, derived from the rabies virus large protein. The research found that RABV-LT induces robust T-cell immune responses and provides partial protection when used alone. The RABV-G-LT combination vaccine elicited sustained neutralizing antibody and T-cell responses in mice and non-human primates, achieving rapid viral clearance, reduced neurological damage, and significantly enhanced protection against lethal infection, demonstrating superior preventive efficacy and longer-lasting protection compared to traditional inactivated vaccines.
abinScience provided the critical RABV-G protein (Catalog No. VK584011) for antibody detection in ELISA experiments, playing a key role in evaluating vaccine-induced antibody responses. This ensured the accuracy and consistency of antibody titer measurements, providing an experimental foundation for validating the vaccine's immunogenicity.
Title: Targeted Radionuclide Therapy of CLDN18.2-Positive Gastric Cancer with [131I]I-Zolbetuximab: An In Vitro and In Vivo Study
Journal: Molecular Pharmaceutics
Impact Factor: 4.5
Author Affiliation: Shanghai Institute of Applied Physics, Chinese Academy of Sciences
This study combined Zolbetuximab with the radionuclide 131I to create [131I]I-Zolbetuximab, investigating its therapeutic efficacy against CLDN18.2-positive gastric cancer. CLDN18.2, a member of the Claudin family, is abnormally overexpressed in gastric cancer. Zolbetuximab, the first monoclonal antibody targeting CLDN18.2, has shown significant survival benefits in clinical trials when combined with chemotherapy. The researchers found through in vitro and in vivo experiments that [131I]I-Zolbetuximab exhibits high labeling efficiency, good stability, specific binding to CLDN18.2-overexpressing cells, significant tumor growth inhibition, and favorable safety, offering a new strategy for targeted radionuclide therapy of CLDN18.2-positive gastric cancer.
abinScience provided the Zolbetuximab monoclonal antibody (Catalog No. HX126016) used for subsequent radionuclide labeling and a series of in vitro and in vivo functional experiments, laying the material foundation for the study’s success.
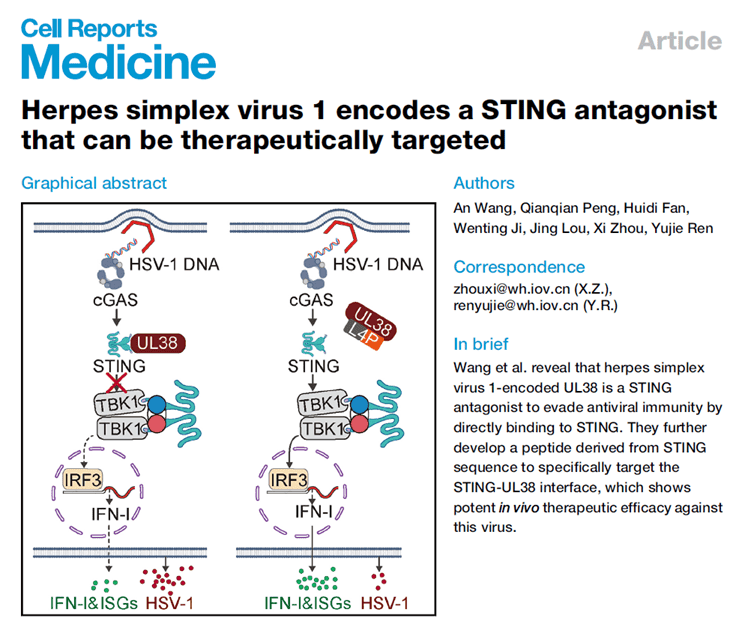
Title: Herpes simplex virus 1 encodes a STING antagonist that can be therapeutically targeted
Journal: Cell Reports Medicine
Impact Factor: 11.7
Author Affiliation: National Key Laboratory of Virology and Biotechnology Safety, Wuhan Institute of Virology, Chinese Academy of Sciences
The study revealed that the HSV-1-encoded UL38 protein is a potent STING antagonist, blocking the cGAS-STING-mediated innate immune response by directly binding to STING and inhibiting its interactions with TBK1 and IRF3. The researchers designed a synthetic peptide, L4P, based on the STING sequence, specifically targeting the UL38-STING binding site. L4P significantly disrupts this interaction, restoring the immune response suppressed by UL38, and demonstrates efficacy comparable to acyclovir in systemic and brain infection models of HSV-1, with good safety. Thus, the study not only elucidates UL38's critical role in HSV-1 immune evasion but also provides a novel antiviral therapeutic strategy.
abinScience provided the rabbit polyclonal antibody against UL38 (Catalog No. VK751014), which played a key role in validating the viral immune evasion mechanism and served as a technical foundation for dissecting the function of HSV-1 UL38.
Literature Citation Reward Program
To express gratitude for the support and trust of researchers, abinScience is launching a Literature Citation Reward Program! Whether you're a pioneer in life sciences or a dedicated laboratory researcher, you can apply for generous rewards by publishing articles in SCI journals using abinScience's full range of products.

 中文
中文 English
English
![Targeted Radionuclide Therapy of CLDN18.2-Positive Gastric Cancer with [131I]I-Zolbetuximab: An In Vitro and In Vivo Study](/storage/20250904/5ded5a2c5e9afe1fe81716e3d2e2fcab.png)
![Targeted Radionuclide Therapy of CLDN18.2-Positive Gastric Cancer with [131I]I-Zolbetuximab: An In Vitro and In Vivo Study (figure)](/storage/20250904/e32fbafd644ec2f0332fcedb642ce761.png)