Pertussis Research in 2025: Epidemiology, Virulence Targets & Vaccine Outlook
U.S. Epidemiology Overview with Local Outbreak Alert
Pertussis, commonly known as whooping cough, is a highly contagious respiratory illness caused by Bordetella pertussis. Globally, it remains a significant vaccine-preventable disease, with an estimated 24.1 million cases and 160,700 deaths in children under 5 years annually based on 2014 modeling estimates (Yeung et al., 2017; CDC, 2024)[1]. In the United States, cyclic epidemics occur every 3–5 years despite high vaccination coverage, driven by waning immunity from acellular pertussis (aP) vaccines and pathogen adaptation. Reported cases trended down since a peak in November 2024, but preliminary 2025 reports remain elevated compared to pre-pandemic levels.[2]
ALERT: Whooping cough cases are on the rise in Wyandotte County, Kansas, and local health officials are advising residents to keep an eye out for symptoms of the illness and stay up to date with their vaccinations.[3] As of late October 2025, at least 15 cases have been confirmed, representing a threefold increase compared to recent years (fewer than 6 cases annually in 2023–2024).[4] Clusters have been noted in schools and daycare centers. Symptoms include prolonged paroxysmal coughing, inspiratory “whoop,” post-tussive vomiting, and apnea in infants. High-risk groups—infants <6 months, pregnant women, and immunocompromised individuals—face severe complications like pneumonia and encephalopathy.
Immunologically, susceptibility stems from declining pertussis toxin (PT)-neutralizing antibodies and impaired Th1/Th17 responses over time, allowing bacterial colonization of ciliated epithelium.
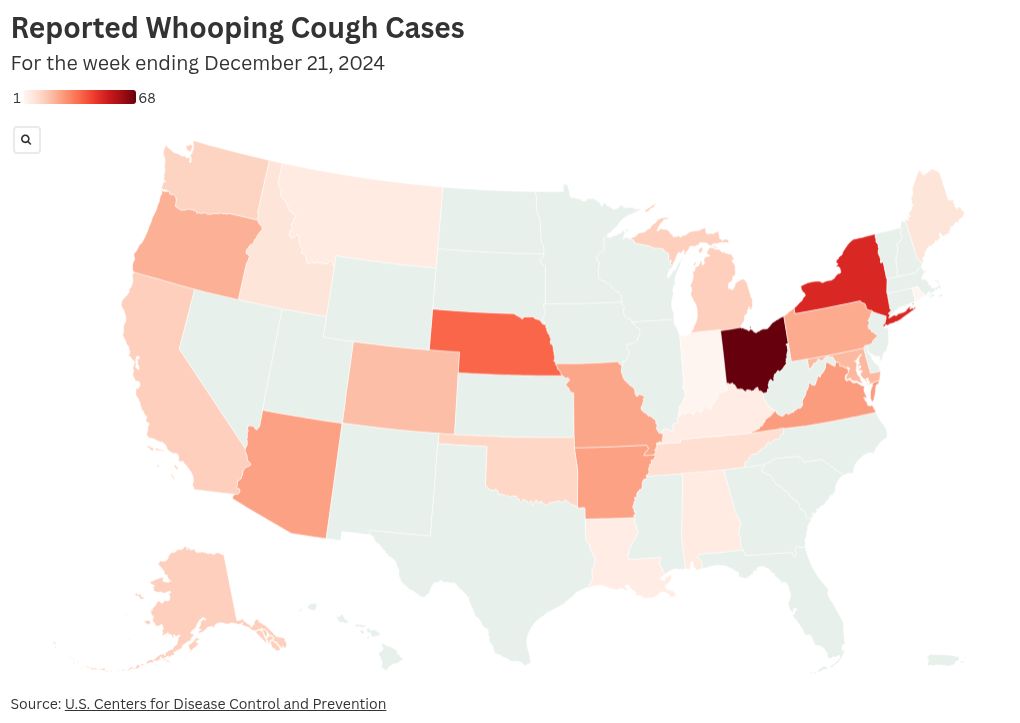
Fig. 1. U.S. Pertussis Incidence Heatmap with Wyandotte County Highlight (2024 YTD )
Pathogen Structure and Immunological Features
The B. pertussis genome is ~4.1 Mb with ~3,800 genes, many dedicated to adhesion, toxin production, and immune modulation. Key virulence factors enable tracheal colonization, ciliary paralysis, and systemic effects. 2025 genomic surveillance reveals increasing prevalence of ptxP3 and prn-deficient strains, linked to enhanced fitness and vaccine escape under aP vaccine pressure, with variations in prevalence across countries.[5]
Pertussis toxin (PT) promotes lymphocytosis and disrupts G-protein signaling; filamentous hemagglutinin (FHA) mediates adhesion; adenylate cyclase toxin (ACT) impairs phagocyte function via binding to CD11b/CD18 (CR3) integrin, elevating cAMP to inhibit chemotaxis, oxidative burst, and phagosome maturation; tracheal cytotoxin (TCT) damages ciliated cells. These factors synergize to establish persistent infection and drive intense coughing for transmission. For PRN-deficient strains, phenotypic compensation occurs through enhanced synergy with FHA and fimbriae (Fim) for adhesion and immune evasion, maintaining virulence despite antigen loss.[6]
Key Targets and Their Functions with Applications:
| Target | Function and Immunological Features | Application Scenarios |
|---|---|---|
| Pertussis Toxin (PT) | S1–S5 subunit holotoxin; ADP-ribosylates Gi proteins, causing lymphocytosis, histamine sensitization, and insulin secretion; primary neutralizing target but rapidly waning antibody target post-aP vaccination | Serological diagnostics (anti-PT IgG); vaccine efficacy biomarker; toxin neutralization assays |
| Filamentous Hemagglutinin (FHA) | Major adhesin; binds integrins and ciliated epithelium; induces anti-adhesive antibodies but also tolerogenic responses; highly immunogenic in aP vaccines | Adhesion inhibition studies; mucosal vaccine design; complement-mediated opsonization research |
| Adenylate Cyclase Toxin (ACT/CyaA) | Bifunctional toxin with adenylate cyclase and hemolysin domains; binds CD11b/CD18 to elevate cAMP in phagocytes, inhibiting chemotaxis, oxidative burst, and phagosome maturation; key for early immune suppression | Innate immunity evasion studies; host-directed therapy targets; macrophage infection models |
| Pertactin (PRN) | Autotransporter adhesin; promotes resistance to neutrophil killing; prn-deficient isolates rising globally, associated with aP vaccine pressure; compensated by FHA/Fim synergy for adhesion/immune evasion | Vaccine escape surveillance; genotyping panels; adhesion mechanism validation |
| Fimbriae (Fim2/3) | Serotype-specific pili; mediate initial attachment; antibodies correlate with short-term protection; antigenic shift observed in circulating strains | Serotyping diagnostics; multi-valent vaccine formulation; agglutination assays |
| Tracheal Cytotoxin (TCT) | Peptidoglycan fragment; selectively kills ciliated cells, causing mucus stasis and prolonged coughing; synergizes with ACT for tissue damage | Airway pathology models; ciliostasis assays; therapeutic target screening |
Recent studies highlight how ACT elevates intracellular cAMP to suppress phagocytic functions and impair phagosome maturation in macrophages, promoting bacterial persistence. These insights drive next-generation vaccines incorporating stabilized PT and novel adjuvants to restore durable Th1/Th17 memory.
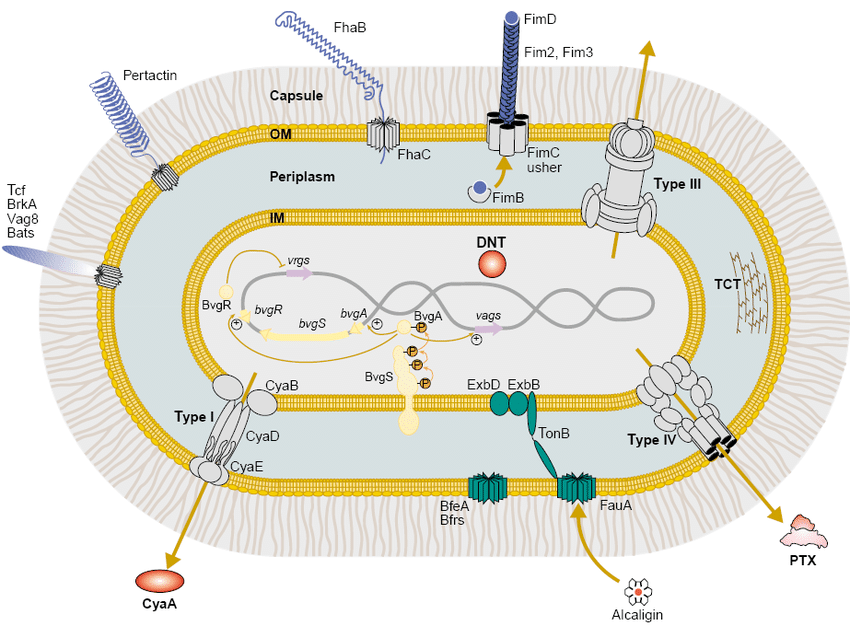
Fig. 2. Schematic of B. pertussis virulence factors. Adapted concept from Melvin JA et al., Nat Rev Microbiol 2014;12:274–288. Used here for educational context.
Challenges in Prevention and Vaccine Development
- Diagnostic Challenges: Early catarrhal phase mimics viral illness; recommended samples include nasopharyngeal swab/aspirate for PCR (optimal 0–3 weeks post-onset) or culture (<2 weeks); serology (anti-PT IgG) preferred ≥3–4 weeks but limited in recently vaccinated individuals.[7]
- Treatment Bottlenecks: Macrolides (azithromycin) effective only in catarrhal/early paroxysmal phase; supportive care dominates in infants. Macrolide-resistant cases (23S rRNA mutations) have been sporadically reported in the U.S., though overall rare; ongoing strain and genotyping surveillance is essential.[2]
- Vaccine Challenges: aP vaccines (DTaP/Tdap) replaced wP in 1990s due to reactogenicity but induce shorter-lived immunity (4–12 years), with aP-induced immunity waning over time (see CDC trends).[2] BPZE1 live attenuated nasal vaccine has completed multiple Phase II studies and Phase IIb challenge trial registration, demonstrating good immunogenicity and safety; Phase IIb colonization endpoint results are pending formal publication.[8]
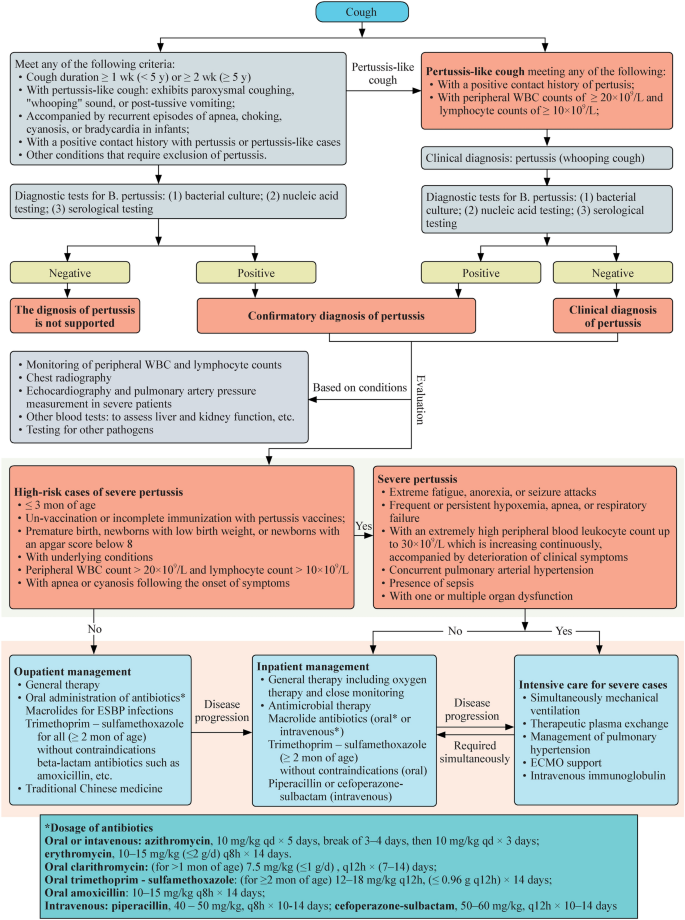
Fig. 3. Pertussis Transmission and Intervention Timeline
Research Application Scenarios
Aligned with 2025 pertussis research priorities, key targets support:
- Immunodiagnostic Development: PT/FHA dual-antigen ELISAs for seroprevalence and outbreak confirmation.
- Vaccine Escape Surveillance: PRN/Fim genotyping to monitor antigenic drift.
- Mucosal Immunity Studies: FHA and ACT in nasal challenge models for next-gen vaccine testing.
- Therapeutic Screening: ACT inhibitors to restore phagocyte function.
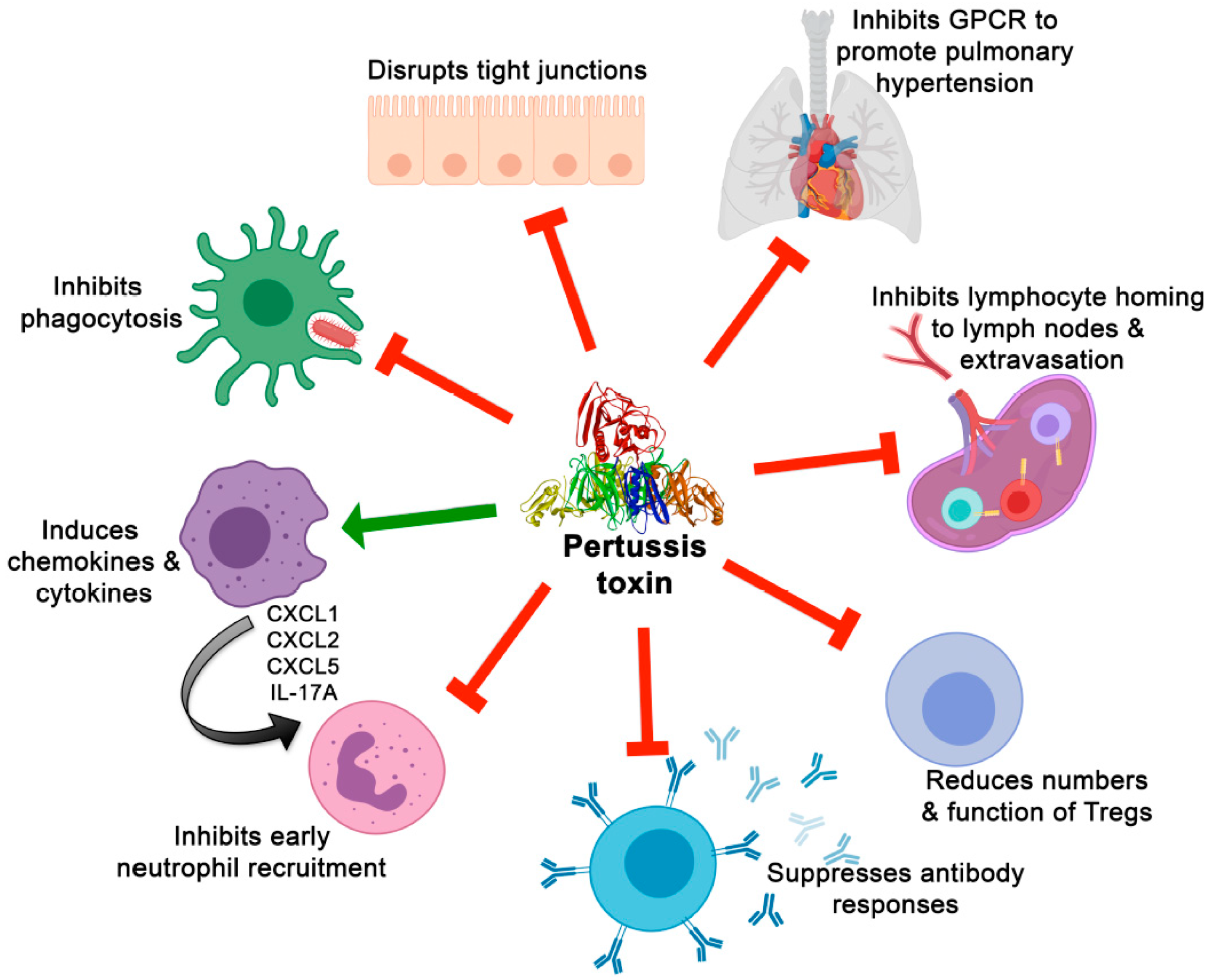
Fig. 4. Applications of Key Antigens in Pertussis Research. Adapted from Scanlon K, et al. Toxins. 2019;11(7):373. doi:10.3390/toxins11070373 (CC BY 4.0)
Pertussis Product Catalog
High-purity recombinant proteins and monoclonal antibodies for pertussis research:
| Type | Catalog No. | Product Name |
|---|---|---|
| Protein | JN113012 | Recombinant Bordetella pertussis dnt3 Protein, N-His |
| JN030012 | Recombinant Bordetella pertussis fhaB/FHA Protein, N-His | |
| JN114012 | Recombinant Bordetella pertussis pagL Protein, N-His | |
| JN115012 | Recombinant Bordetella pertussis prn/P.94 Protein, N-His | |
| JN968012 | Recombinant Bordetella pertussis ptxA/PTXS1 Protein, N-His | |
| Antibody | JN113014 | Anti-Bordetella pertussis dnt3 Polyclonal Antibody |
| JN030013 | Anti-Bordetella pertussis fhaB/FHA Antibody (T5) | |
| JN030014 | Anti-Bordetella pertussis fhaB/FHA Polyclonal Antibody | |
| JN114014 | Anti-Bordetella pertussis pagL Polyclonal Antibody | |
| JN115014 | Anti-Bordetella pertussis prn/P.94 Polyclonal Antibody | |
| JN968014 | Anti-Bordetella pertussis ptxA/PTXS1 Polyclonal Antibody | |
| JN987010 | InVivoMAb Anti-Bordetella pertussis cyaA/Cyclolysin Antibody (Iv0144) | |
| JN987020 | InVivoMAb Anti-Bordetella pertussis cyaA/Cyclolysin Antibody (Iv0145) |
About abinScience
abinScience is a biotechnology company headquartered in France, focused on infectious disease research and public health innovation. We develop high-quality, research-grade tools to empower scientists worldwide in the fight against pertussis — from rapid outbreak detection and strain surveillance to next-generation vaccine design and immune mechanism studies.
Empowering Bioscience Discovery
References
- Yeung KH, et al. (2017). An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis, 17(9):974-980.
- Centers for Disease Control and Prevention. (2025). Pertussis Surveillance and Trends. https://www.cdc.gov/pertussis/php/surveillance/index.html.
- Wyandotte County Health Department. (2025). Health Officials Warn of Rise in Whooping Cough Cases. Link.
- Kansas City Star. (2025). Whooping cough surge in Wyandotte County. Link.
- Safarchi A, et al. (2015). Pertactin negative Bordetella pertussis demonstrates higher fitness under vaccine selection pressure in a mixed infection model. Vaccine, 33(46):6277–6281. doi: 10.1016/j.vaccine.2015.09.064.
- Kroes MM, et al. (2021). Naturally circulating pertactin-deficient Bordetella pertussis strains induce distinct gene expression and inflammatory signatures in human dendritic cells. Emerg Microbes Infect, 10(1):1358-1368. doi: 10.1080/22221751.2021.1943537.
- CDC. (2025). Laboratory Testing for Pertussis. https://www.cdc.gov/pertussis/php/laboratories/index.html.
- Faust SN, et al. (2025). Immunogenicity and Safety of BPZE1 in Children. Open Forum Infect Dis, 12(Suppl 1).
- Melvin JA, et al. (2014). Bordetella pertussis pathogenesis: current trends and future prospects. Nat Rev Microbiol, 12(4):274–288. doi:10.1038/nrmicro3223.
- Scanlon K, et al. (2019). The Structure, Function and Regulation of Bordetella pertussis Virulence Factors. Toxins, 11(7):373. doi:10.3390/toxins11070373.

 中文
中文 English
English


