The World Health Organization has just released an update—by mid-August 2025, 31 countries have reported over 400,000 cases of cholera/acute watery diarrhea, with nearly 5,000 deaths. The hardest-hit regions are Africa and the Eastern Mediterranean, with the Democratic Republic of Congo reporting nearly 47,000 cases, South Sudan over 71,000 cases, and case fatality rates in Chad and the Republic of Congo reaching as high as 6–8%, far exceeding the alert threshold. Medical treatment and prevention systems are under immense pressure.
Cholera is an acute intestinal infectious disease caused by Vibrio cholerae. It spreads rapidly through contaminated water and food. If not treated promptly with rehydration, patients can die within hours due to severe dehydration, with organs failing one by one, leaving the body resembling a bluish, emaciated corpse. Cholera is not only a scientific issue but also a social problem tied to poverty, conflict, and inadequate infrastructure.
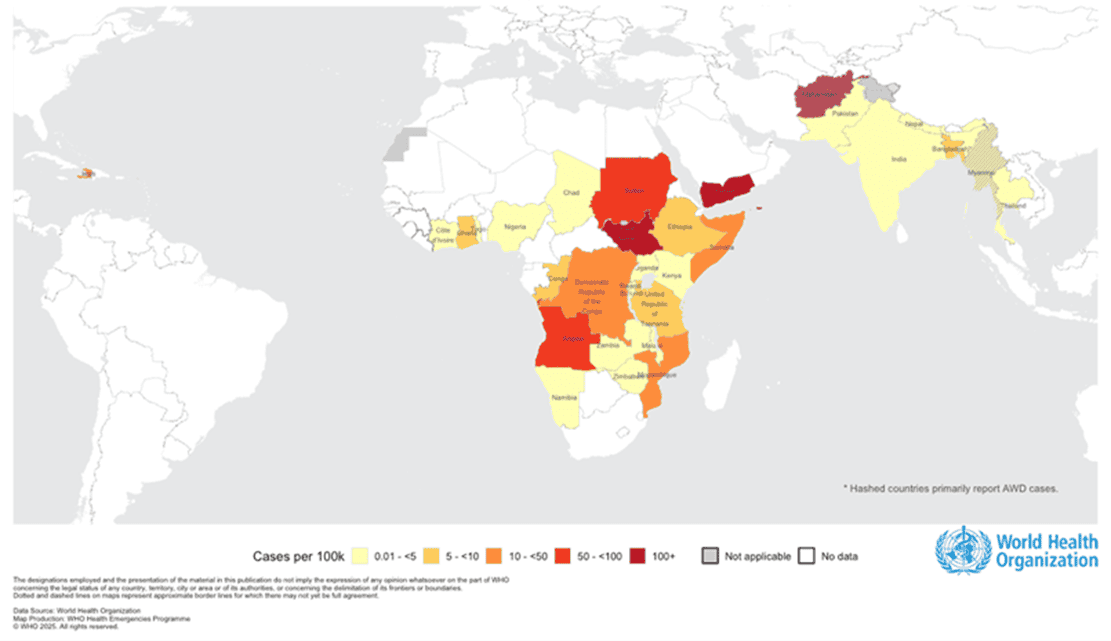
Figure 1. Global cholera and acute watery diarrhea (AWD) cases per 100,000 people (January 1 to August 17, 2025, data source: WHO)
Structure of Vibrio cholerae
Vibrio cholerae is a Gram-negative facultative anaerobic bacterium with a short, comma-shaped body. Its outer layer consists of a typical lipopolysaccharide (LPS) structure, where the O-antigen determines the serotype (O1 and O139 are most commonly associated with large-scale outbreaks).
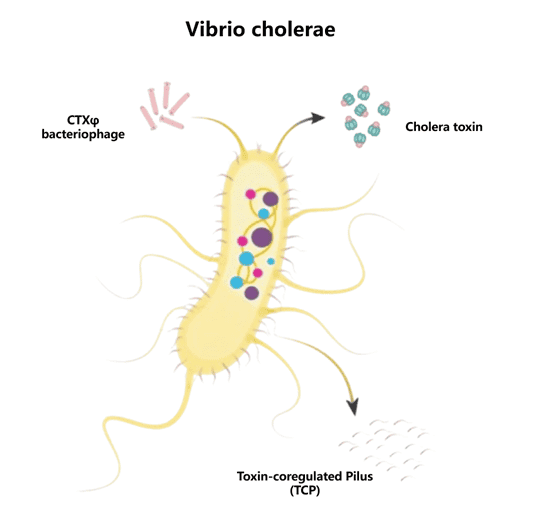
Figure 2. Schematic diagram of Vibrio cholerae structure
Major Virulence Factors
- Cholera Toxin (CTX): Encoded by the ctxAB genes on the CTXφ bacteriophage genome. The A subunit catalyzes ADP-ribosylation, continuously activating G proteins, leading to increased adenylate cyclase activity, elevated cAMP levels, and massive secretion of water and electrolytes, causing severe diarrhea. The B subunit, a pentamer, binds to GM1 gangliosides on the host cell membrane, facilitating toxin entry.
- Zot (Zonula occludens toxin): Disrupts tight junctions in the intestinal epithelium, increasing intestinal permeability and exacerbating fluid loss.
- Ace (Accessory cholera enterotoxin): An auxiliary enterotoxin that intensifies diarrhea by stimulating chloride ion secretion.
- Hap (Haemagglutinin/protease): Involved in adhesion, protein degradation, and colonization.
- RTX Toxin: A cytotoxin that disrupts the cytoskeleton and interferes with the host immune response.
Pathogenesis
Cholera Toxin (CTX) is the core weapon of Vibrio cholerae. Its B subunit first binds to GM1 gangliosides on the host cell surface. If GM1 is absent, the toxin may bind to other glycans, such as Lewis Y and Lewis X, attached to proteins rather than lipids. The toxin is then internalized by the cell, entering the Golgi apparatus and endoplasmic reticulum. Here, disulfide bonds are cleaved, releasing the catalytically active A1 chain. The A1 chain is translocated into the cytoplasm via the endoplasmic reticulum, where it refolds and ADP-ribosylates the α subunit of the trimeric G protein (Gsα), keeping it in an active state. This results in continuous activation of adenylate cyclase, significantly elevated cAMP levels, and massive secretion of water and electrolytes by intestinal cells, leading to the characteristic "rice-water diarrhea," causing severe dehydration and electrolyte imbalances.
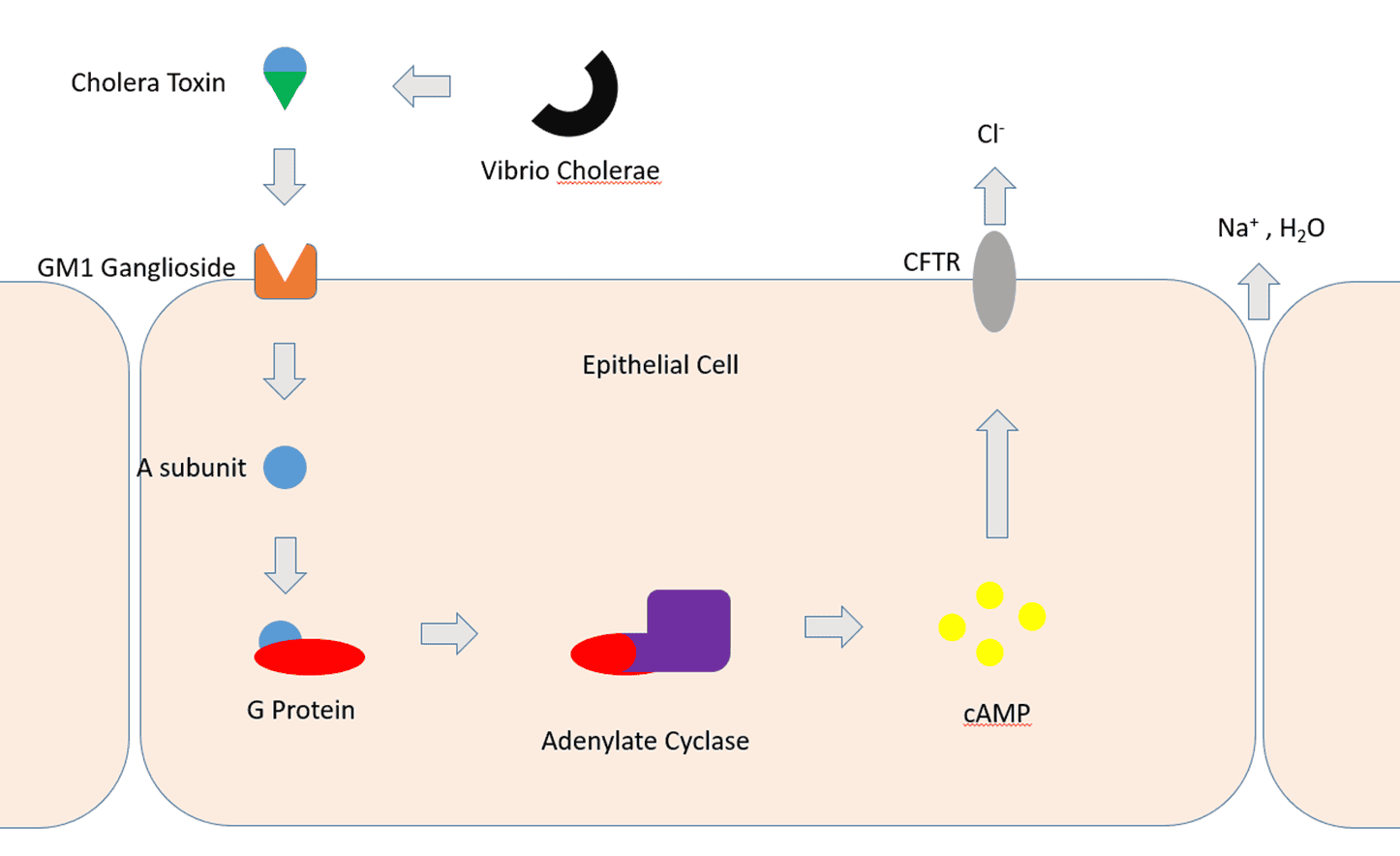
Figure 3. Pathogenesis of cholera toxin
(Image source: Author Mclaneb1)
Vaccine Progress
| Vaccine Type |
Characteristics |
| Existing OCV (Shanchol/Euvichol) |
Two-dose regimen with long-lasting protection; single-dose strategy used in emergencies; limited stockpile with no alternative manufacturers |
| CCV (Conjugate Vaccine) |
Targets children with stronger, long-lasting protection; supported by funding, still in development |
| HaitiV (Live Vaccine) |
Single-dose with rapid onset and potential for long-term protection, in preclinical stage |
| Hillchol (New Oral Vaccine) |
Phase III success, strong immunity, low cost, high production capacity, expected to alleviate supply shortages |
Public Health Significance of Cholera
In China, cholera is classified as a Class A infectious disease, the highest level of statutory infectious disease management. By comparison, COVID-19, which we recently experienced, is managed as a Class B disease in China, highlighting the severity of cholera. It spreads through multiple routes (water, food, and contact), progresses rapidly, and can be fatal within hours due to dehydration if untreated. The public health practices we were taught from a young age—such as "boiling water" and "washing hands before meals and after using the restroom"—are highly necessary from a public health perspective. Boiling water effectively kills most pathogenic bacteria, including Vibrio cholerae, while good hand hygiene significantly reduces the risk of oral transmission, preventing diseases from “entering through the mouth.”
abinScience Research Support
For this reason, fundamental research and prevention products for Vibrio cholerae are critical. abinScience has provided antibodies and recombinant protein products for numerous studies, aiding researchers in analyzing virulence factors, signaling pathways, and host responses. Founded in Strasbourg, France, abinScience leverages the region's exceptional research and innovation ecosystem to focus on the development and production of high-quality life science reagents. Committed to the vision of "Empowering Bioscience Discovery," abinScience strives to provide efficient and reliable experimental solutions to researchers worldwide, enabling cutting-edge life science research.
Below is a list of Vibrio cholerae-related protein and antibody products offered by abinScience:
| Type |
Catalog No. |
Product name |
| Protein |
JN908012 |
Recombinant Vibrio cholerae serotype O1 DnaA Protein, C-His |
| JN004012 |
Recombinant Vibrio cholerae serotype O1 RctB Protein, N-His |
| JN074012 |
Recombinant Vibrio cholerae serotype O1 ctxB/Cholera Toxin Subunit B Protein, N-His-SUMO |
| JN074022 |
Recombinant Vibrio cholerae serotype O1 ctxB/Cholera Toxin Subunit B Protein, N-His |
| Antibody |
JN074014 |
Anti-Vibrio cholerae serotype O1 ctxB/Cholera Toxin Subunit B Polyclonal Antibody |
| JN074013 |
Anti-Vibrio cholerae ctxB/Cholera Toxin Subunit B Nanobody (A9) |
| JN074023 |
Anti-Vibrio cholerae ctxB/Cholera Toxin Subunit B Antibody (TE33) |
| JN067113 |
Anti-Vibrio cholerae LPS/Lipopolysaccharide Antibody (SAA0582) |
| JN074033 |
Anti-Vibrio cholerae ctxB/Cholera Toxin Subunit B Antibody (SAA0848) |
| JN074043 |
Anti-Vibrio cholerae ctxB/Cholera Toxin Subunit B Antibody (SAA0849) |
| JN080013 |
Anti-Vibrio cholerae higB-2 Nanobody (SAA0850) |
| JN080023 |
Anti-Vibrio cholerae higB-2 Nanobody (SAA0851) |
| JN080033 |
Anti-Vibrio cholerae higB-2 Nanobody (SAA0852) |
| JN074053 |
Anti-Vibrio cholerae serotype O1 ctxB/Cholera Toxin Subunit B Nanobody (SAA1345) |
| JN080043 |
Anti-Vibrio cholerae serotype O1 higB-2 Nanobody (SAA0877) |
| JN080053 |
Anti-Vibrio cholerae serotype O1 higB-2 Nanobody (SAA1029) |
| JN080063 |
Anti-Vibrio cholerae serotype O1 higB-2 Nanobody (SAA1035) |
| JN080073 |
Anti-Vibrio cholerae serotype O1 higB-2 Nanobody (SAA1174) |

 中文
中文 English
English





