Release date:
2025-11-13 View count: 13
Flow cytometry is one of the most widely used cell detection techniques, commonly applied in immunology, haematology, and clinical testing. Blood is one of the most common sources for flow cytometry samples, as it is a natural single-cell suspension that can be used directly for detection. However, a practical issue arises: the number of red blood cells (RBCs) in blood is significantly higher than that of white blood cells. When the target for analysis is white blood cells (such as lymphocytes, monocytes, and granulocytes), the excessive number of RBCs can interfere with the results, making it difficult to distinguish the target population. This necessitates the processing of blood samples before the experiment, using appropriate methods to remove red blood cells and platelets, thereby facilitating subsequent research.
The common methods for processing blood samples include red blood cell lysis and density gradient centrifugation. So, how should one choose between these two methods for blood sample preparation?
1. Red Blood Cell Lysis
Red blood cell lysis is a commonly used method for processing blood samples in routine experiments. By using a specialised lysis buffer, red blood cells are selectively destroyed, while the majority of white blood cells are retained. After lysis, the sample can proceed to subsequent staining or detection steps.
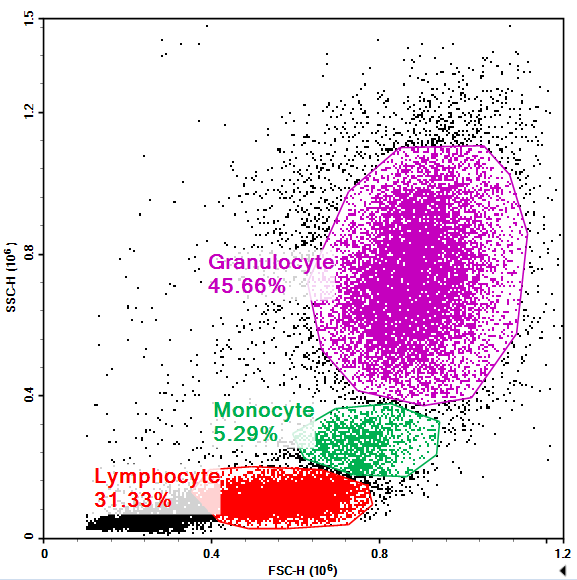
Figure 1. Red blood cell lysis of human peripheral blood
Advantages
- Fast and simple operation: The entire process only takes a few minutes, making it suitable for high-throughput sample processing.
- High white blood cell retention: Lymphocytes, monocytes, and granulocytes are generally well-preserved.
Disadvantages
- Potential reduction in cell viability: Compared to density gradient centrifugation, cell function may be slightly affected during the lysis process.
- Sensitive to operating conditions: Lysis time that is too long may damage white blood cells, while too short a time may result in incomplete lysis, leaving red blood cells behind.
- Limited applications for further experiments: If the sample needs to be cultured or used in functional assays, lysis treatment may cause interference.
Experimental Considerations
- Strict control of time: The process should be completed within the recommended time to avoid the negative impact of prolonged lysis on other cells.
- Target consideration: If the research object involves red blood cells (e.g., anaemia or haemoglobinopathies), lysis should not be used.
- Caution for downstream applications: If planning to culture or freeze cells, lysis treatment may reduce cell viability, requiring prior evaluation.
2. Density Gradient Centrifugation
Density gradient centrifugation separates cell populations based on their sedimentation coefficients and density differences through a gradient medium, such as Ficoll or Percoll.
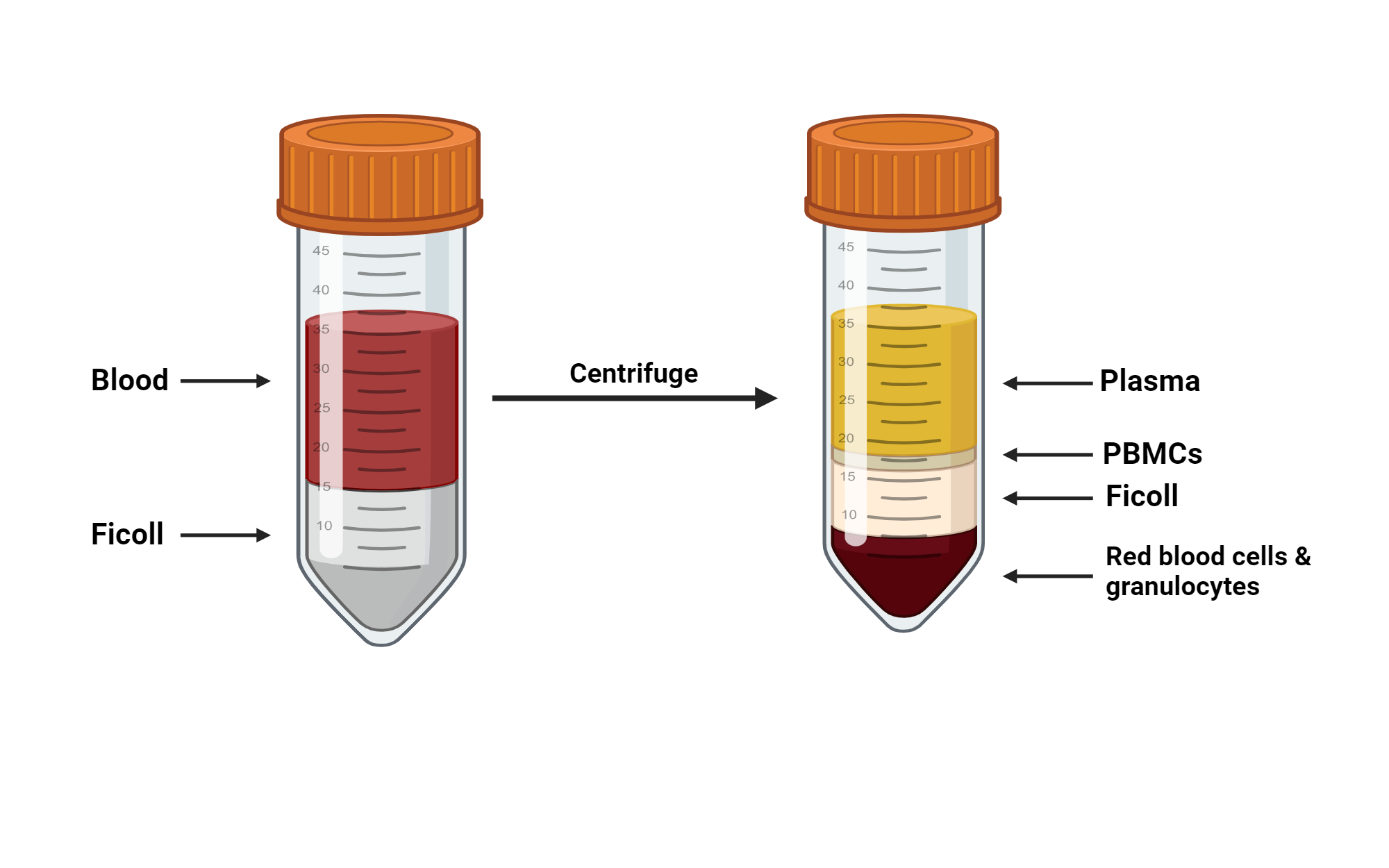
Figure 2. Schematic of PBMC extraction from human peripheral blood using Ficoll separation medium (Image source: online)
Advantages
- High purity: The separation is effective, yielding high-purity cells.
- Better cell viability: This method is suitable for subsequent functional experiments, such as proliferation assays, cytokine secretion detection, and T cell function studies.
Disadvantages
- Complex operation: The process usually takes more than 30 minutes and involves several steps.
- Higher technical skill required: Skilled operators are needed to achieve stable and clear layering.
- Limited white blood cell retention: Some white blood cells, such as neutrophils, may be removed, with only lymphocytes and monocytes retained.
Experimental Considerations
- Smooth centrifugation: Acceleration and deceleration should be gentle to maintain clear separation.
- Species differences: The PBMC densities vary among humans, mice, and rats, so the separation medium cannot be mixed. For humans, PBMC density is 1.077 g/mL, while for mice and rats, it is 1.084 g/mL.
3. Applications of Both Methods
Rapid Whole Blood Immune Profiling
If the goal is to quickly analyse the quantity and surface markers of white blood cell subpopulations in whole blood (e.g., lymphocyte subsets, monocyte count), red blood cell lysis is often the preferred choice. This method is simple and quick, allowing for the processing of large numbers of samples. It is particularly effective for clinical testing or large-scale sample screening, as it efficiently removes red blood cells while retaining most white blood cells for analysis.
High-Purity PBMC Functional Analysis
If downstream experiments require high-purity lymphocyte/monocyte populations (e.g., in vitro cell culture, cytotoxicity assays, cytokine detection), density gradient centrifugation is more suitable. This method yields relatively pure PBMC populations and best preserves cell viability. However, it should be noted that some white blood cells (such as small numbers of lymphocytes or cell debris) may be lost during the process, and neutrophils are removed.
Specific Cell Type Requirements
If the research requires the preservation and analysis of neutrophils or other dense white blood cell populations (such as granulocyte function studies), red blood cell lysis should be used. If only lymphocytes and monocytes are of interest, density gradient centrifugation offers a higher-purity sample.
4. Conclusion
In summary, the choice of pre-treatment method should be based on the experimental objectives and subsequent analysis needs. If the focus is on rapid screening of white blood cell subsets in whole blood or when the sample size is large and purity requirements are modest, red blood cell lysis is the preferred option due to its simplicity and efficiency. If high-purity PBMCs are needed for cell culture and functional assays, density gradient centrifugation is better suited to ensure higher purity and better cell viability. It is important to note that no single method is universally applicable, and factors such as ease of operation, cell purity, and viability should be considered in the decision. In practice, both methods can also be combined; for example, red blood cells can be quickly removed by lysis, followed by gradient separation of specific cell populations for optimal results.
About Us
As a strategic venture of AtaGenix (established 2011), abinScience was founded in 2023 to deliver premium life science reagents that accelerate discovery. Our flow cytometry antibody products cover commonly used detection markers, with a wide variety to meet the research needs of multiple species (Human, Mouse, Rat, Dog, Hamster, Monkey, etc.). We provide stable and reliable support for scientific research. For more information on abinScience flow cytometry antibodies, please click:
abinScience Flow Cytometry (FACS) Antibodies

 中文
中文 English
English




