Release date:
2025-11-17 View count: 30
In multicolour flow cytometry experiments, "compensation" is often perceived as a result automatically calculated by software. As long as the algorithm is correct and the channels are set appropriately, the data seems to be error-free. However, the true factor determining the accuracy of the compensation matrix lies in an earlier step—the quality of the single-stain control signal. A clean, appropriately bright, and well-separated single-stain control is not only the foundation for algorithmic calculation but also the starting point for ensuring the credibility of experimental data.
1. Compensation Matrix Accuracy Determines Data Credibility
In multicolour flow cytometry, spectral overlap between different fluorophores is inevitable (Figure 1). The compensation algorithm can correct this "spillover", but its accuracy entirely depends on the quality of the single-stain control signal.
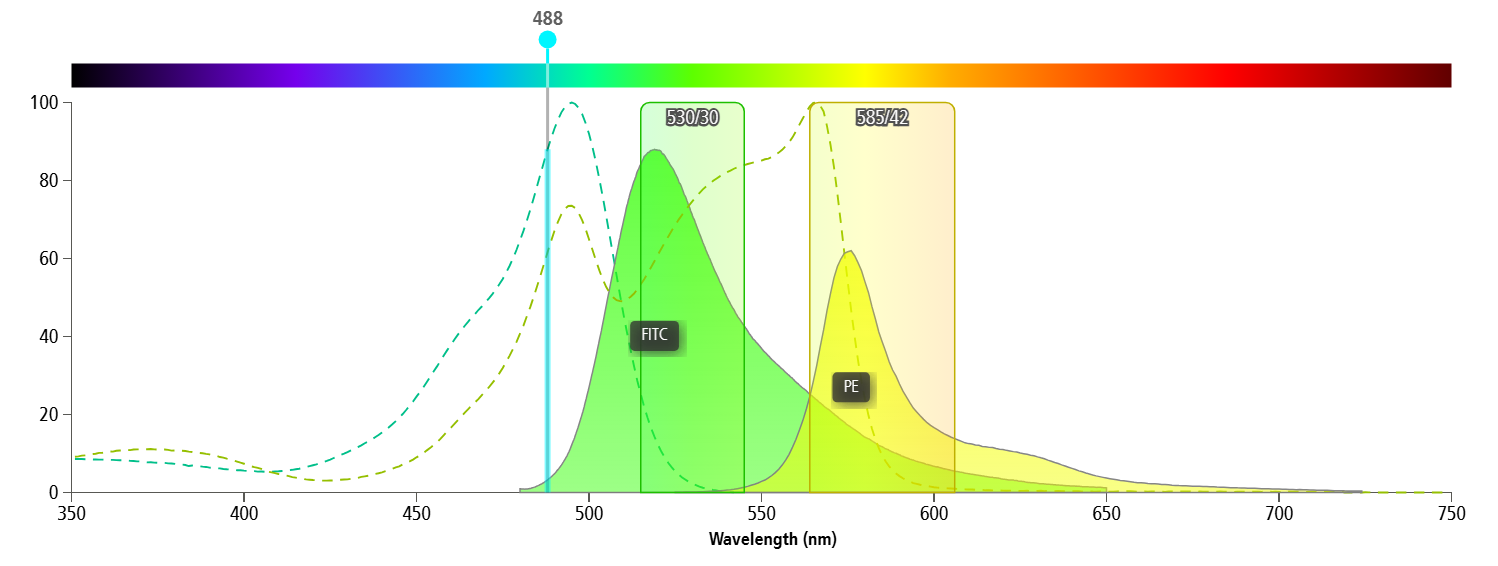
Figure 1: Spectral overlap
In many cases of compensation shift or data overlap, the issue does not lie with the software, but rather with the "poor quality of the single-stain signal"—insufficient brightness, fuzzy population, or mismatched samples and panel.
2. Single-Stain Controls Are Not Just "Formality", But "Signal Standards"
The single-stain control defines the pure fluorescence signal for each channel and is the sole basis for compensation matrix calculations. Therefore, it is not just about having a control, but it must meet the following conditions:
1) Appropriate Signal Brightness: The positive population signal should be sufficiently above the background but should not exceed the detection range.
2) Clear Distinction Between Positive and Negative Populations: This can be evaluated using Staining Index (SI) or signal-to-noise ratio.
3) Sample Matching: The single-stain control must match the whole-stain control in terms of cell type, fixation/permeabilisation conditions, and voltage settings.
4) Adequate Positive Population: Ensure that the software can detect positive events, which are used for matrix calculation.
These parameters together determine the "stability and reproducibility" of the compensation matrix, rather than merely "channel correction".
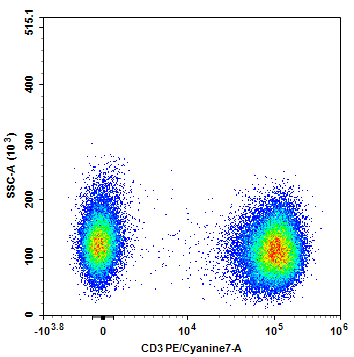
Figure 2: CD3 single-stain control signal
3. How to Assess the Quality of Single-Stain Controls?
The quality of the single-stain signal can be assessed based on the following characteristics:
|
Detection Point
|
Ideal Condition
|
Common Issues
|
Potential Consequences
|
|
Positive Signal Intensity
|
Significantly higher than negative population
|
Signal too weak or too bright
|
Compensation shift or matrix distortion
|
|
Population Boundary
|
Clear division between positive and negative populations
|
Mixed or indistinct boundaries
|
Software failure to recognise
|
|
Negative Population Position
|
Stable distribution
|
Negative population drifting
|
Negative compensation
|
|
Cell Condition
|
Stable FSC/SSC
|
High proportion of dead cells
|
Non-specific signal interference
|
Practical Tips:
1) For low-expression markers (such as IL-17A, Foxp3), use high-expression markers (e.g., CD3, CD4) with the same fluorophore to create a single-stain control.
2) For fluorochrome-conjugated tandem dyes (e.g., PE-Cy7, APC-Cy7), use compensation beads instead of cells to avoid signal abnormalities due to dye degradation.
4. Signal Issues ≠ Compensation Issues
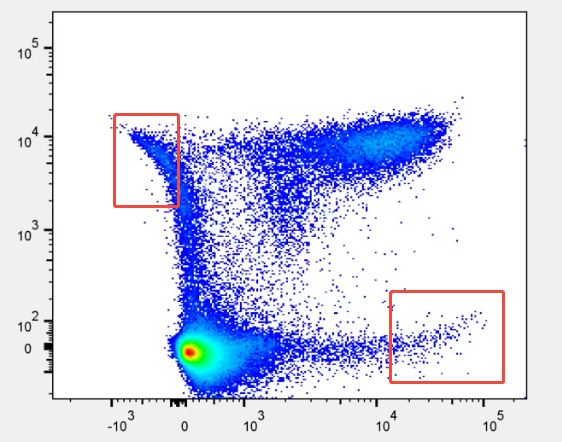
Figure 3: Experiment results showing signal skew
When populations do not align, data drifts, or the positive and negative populations appear skewed, the direct cause is typically improper compensation settings, either too high or too low. However, a deeper analysis often reveals that the real cause of this compensation error originates from poor single-stain signal quality or inconsistent experimental conditions—such as weak positive populations, unclear population boundaries, or variations in sample handling—leading to errors in the baseline signal used for compensation matrix calculations.
In other words, what seems to be a compensation algorithm issue is, in fact, a result of the single-stain signal not being "clean" enough, leading to inaccurate compensation settings.
Common Misconceptions:
1) Mistaking signal issues for compensation errors: When data shifts, the immediate response is to recalculate the matrix, neglecting whether the single-stain signal itself is clean.
2) Single-stain signal too weak or too bright: A weak positive population can prevent the software from recognising the positive/negative boundary, while an overly bright signal leads to signal overflow, both causing compensation discrepancies.
3) Single-stain and whole-stain conditions are inconsistent—different sample types, voltage settings, or fixation/permeabilisation methods can alter fluorescence intensity, distorting compensation results.
4) Tandem dye degradation goes unnoticed: For example, PE-Cy7 or APC-Cy7 degradation causes abnormal fluorescence, often resulting in compensation values greater than 100%.
5) Overlooking channel contamination or negative population drift: This is especially problematic when using the same sample tubes repeatedly or when voltage fluctuates, potentially leading to negative compensation.
Therefore, the first step in troubleshooting compensation errors should always be to re-examine the single-stain signal, rather than recalculating the matrix. Ensuring that the signal itself is "clean and reliable" is often more effective than any algorithmic correction.
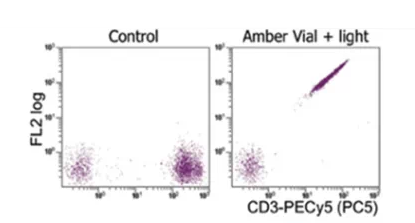
Figure 4: Tandem dye degradation leading to signal anomalies
5. Practical Tips for More Stable Compensation
1) Panel Planning: Choose fluorophores with low spectral overlap to reduce compensation demands.
2) Prioritise Signal Quality: It's better for the single-stain signal to be slightly brighter than too faint.
3) Ensure Consistency: Ensure that single-stain and whole-stain controls are fully matched in terms of sample type, voltage, and fixation/permeabilisation conditions.
4) Use Magnetic Beads Appropriately: For tandem dyes, difficult-to-isolate positive populations, or insufficient sample volume, magnetic beads can be used to enhance signal stability.
5) Regular Quality Control: Check the compensation matrix after each experiment. If compensation values exceed 100% or show negative compensation, trace back to the single-stain signal.
6. Practical Example of a Single-Stain Control Setup
Here is an example of a single-stain control setup for a four-colour flow cytometry experiment designed to detect the Treg cell subset in human peripheral blood mononuclear cells (PBMCs). The antibodies include CD3-PerCP, CD4-FITC, CD25-APC, and Foxp3-PE. We need to set up four single-stain controls (Table 1).
Table 1: Single-Stain Control Setup Reference Table
|
No.
|
Antibody Added
|
Sample Treatment
|
|
1
|
CD3-PerCP
|
Requires fixation/permeabilisation
|
|
2
|
CD4-FITC
|
Requires fixation/permeabilisation
|
|
3
|
CD25-APC
|
Requires fixation/permeabilisation
|
|
4
|
Foxp3-PE
|
Requires fixation/permeabilisation
|
Note:
1) All single-stain controls use the same PBMC batch and instrument settings as the whole-stain samples (e.g., BD FACSCanto II), with voltage adjusted so the positive signal falls within the 10⁴-10⁵ range. At least 10,000 events are collected per tube.
2) CD25 is continuously expressed, while Foxp3 is low-expressed. These can be replaced with high-expression markers using the same fluorophore, such as using CD3-APC instead of CD25-APC, or CD3-PE instead of Foxp3-PE. The substituted markers are extracellular, or compensation beads with corresponding antibodies can be used.
3) Since Foxp3 is an intracellular transcription factor, the four single-stain controls should undergo fixation/permeabilisation, regardless of whether high-expression markers or compensation beads are used.
This example demonstrates the setup and verification process for single-stain controls, emphasising the importance of signal strength, population clarity, and consistency in experimental conditions to ensure a reliable compensation matrix.
Single-stain controls are the most easily overlooked yet most decisive step in multicolour flow cytometry. A clean, clear, and stable single-stain signal is the prerequisite for building a reliable compensation matrix. A distorted compensation matrix can render the entire dataset uninterpretable. In increasingly complex multicolour panel designs and high-throughput analyses, returning to signal quality and focusing on single-stain controls remains central to ensuring experimental stability and data reliability. To ensure each compensation is "stable", it all starts with the single-stain control.
About Us
As a strategic venture of AtaGenix (established 2011), abinScience was founded in 2023 to deliver premium life science reagents that accelerate discovery. Our flow cytometry antibody products cover commonly used detection markers, with a wide variety to meet the research needs of multiple species (Human, Mouse, Rat, Dog, Hamster, Monkey, etc.). We provide stable and reliable support for scientific research. For more information on abinScience flow cytometry antibodies, please click:
abinScience Flow Cytometry (FACS) Antibodies

 中文
中文 English
English






